Track & Trace has been on everyone's lips for some time now, but what is it actually about? It usually means the identification of individual products, the traceability of production and quality data and the sales channels. Prerequisites for the implementation of Track & Trace are the labelling of the products, suitable camera and identification systems, along with the availability of the data.

What is "Track & Trace"?
The term Track & Trace originally comes from the logistics sector. First of all, it is used to describe the traceability of shipments: when was a package picked up, when did it arrive at the destination airport, etc... Now, in a very short time, the term has become a key word that is also used to mean other things. It also means the traceability of different stations in production, raw material procurement, production and quality data and sales channels. Track & Trace therefore refers to the entire "supply chain".
Prerequisites and Goals
A first requirement for Track & Trace is the clear identification of a product. In the case of mass-produced products, this is done by marking the product itself (direct part marking) or, where this is not possible or is too time-consuming, via its packaging. The marking is applied by the manufacturer and must be able to be read again reliably at a later time anywhere in the world. Data can now be collected and stored for each individual product. This data must be available and retrievable when needed. A driving force in traceability is the pharmaceutical industry, where so-called serialisation aims at identification down to the individual medicine box.
There are several reasons why companies today rely on Track & Trace. Important aspects are counterfeit protection, product liability and the traceability of the routes a product takes in the distribution chain. Of course, further optimisations are possible through the online collection of production data.


Type of marking
Lettering in plain text is the traditional type of marking. It has the advantage that it can be read again by people without any tools, but it is less suitable for automatic identification. Nevertheless, there are solutions from specialists that are suitable for the industrial environment, as shown by the example with the containers. Here, the goal is to identify the contents of a container by means of the labelling on the document panel. This is done with a camera system and reading software (OCR for Optical Character Recognition), which extracts and reads the characters from the image.
The most common method of marking for machine identification is still the linear barcode. More and more often, readers are equipped with imaging sensors. Compared to laser scanners, newer generation devices offer improved reading reliability for damaged codes and more flexibility, as with these devices the code may be placed anywhere in the camera image and rotated. However, the information content of linear codes is rather modest with relatively large space requirements.
Two-dimensional codes are a proven further development. These make much better use of the available space and their information content is massively greater with a smaller footprint. In addition, these codes can be read much more reliably because they have a large redundancy that is used in error correction procedures. Even damaged codes can still be read reliably. For 2D codes, the DataMatrix Code ECC200 has become firmly established and is now even required by law in the pharmaceutical industry for labelling on packaging.
In addition, there are other identification methods such as RFID or proprietary methods. However, applying a code on a label or on a product is generally the simplest and cheapest way of identification.

Standards
The linear barcodes are defined in the international standard ISO/IEC 15420 and the two-dimensional DataMatrix code in the standard ISO/IEC 16022.
When checking the print quality, the so-called verification, the standards ISO/IEC 15415 and 15416 are applicable. If codes are not printed but applied by other means, other standards such as AIM DPM or AS9132 can also be considered.
The content or the structure of the data is defined in the GS1 General Specifications (GS1 is the successor organisation of EAN). The ANS_MH10.8.2 standard determines how a code must be structured in terms of content. Individual fields are identified with "Application Identifiers" (AI) and the content of these fields must also be compliant. Common AIs are GTIN (Global Trade Item Number, with which products can be uniquely identified worldwide), lot number, expiry date, etc.
Code Verification and Validation
Verification is about assessing the quality of the applied code. This is to ensure that the codes can be reliably read again at any time with readers from different manufacturers. The ISO/IEC standards 15415 and 15426 define how quality is to be measured and are well suited for imprints on folding boxes. For direct part marking of metal parts, for example, this standard is rather unsuitable. In the automotive industry, for example, the AIM DPM standard is therefore preferred.
The code is validated to check its conformity with the GS1 standard. The code is read, the individual AIs and the associated fields are identified and then checked for correctness. Some countries have already adopted additional regulations for some time, which then require a country-specific check.
The "DataMatrix Code Verifier" allows verification according to ISO/IEC 15415 as well as GS1 - validation for carton sections or entire folding boxes. The standard requires that the code is checked and evaluated in five specific rotational positions during verification, which is very easy with the help of the device developed and patented by Compar.
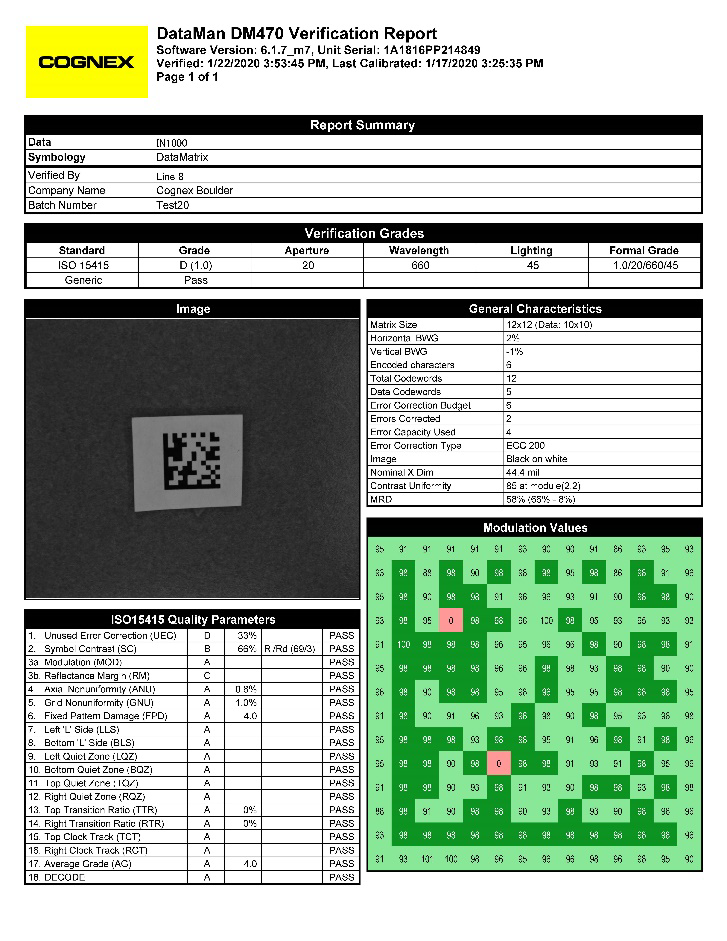

On-line Grading and OCV
For the inspection on the production line, fast systems are needed that combine different functionalities. An example is the verification of a DataMatrix code with subsequent verification of the content printed next to it in plain text. In opposition to the verification according to ISO/IEC 15415, the code is only picked up, read and evaluated in one rotational position. For the verification of the characters, the values are taken from the DataMatrix code and the characters in the corresponding fields are checked for correctness of the information and print quality. Since the text to be checked is already known after the code has been read, this is referred to as Optical Character Verification or OCV.
The purpose of on-line grading is to guarantee consistent quality in production. The conformity with the standards is a must.
Traceability
Nowadays, for typical products such as medicines, it is possible to label each box individually and to archive the production and test data associated with each serial number. If the coding is correct in terms of content and quality, a box can later be taken from the shelf and identified, for example, with the help of a handheld reader. Then it is possible to trace how, when and under which conditions the product was produced and tested and through which channels it was sold.
The supplier of the test systems is able to record his test requirements and the results of the quality tests in production in a tamper-proof manner. If the products are later identified again, the manufacturer is able to provide the necessary evidence in the event of product liability. Furthermore, the traceability of the sales channels will make it easier to put a stop to counterfeiters and other criminal elements.

The possibilities described above are not utopias but are currently being implemented today. Compar was the first partner system integrator of Cognex in Europe and provides innovative solutions in the field of industrial image processing and identification.

